GM mosquito questions still need answers: GeneWatch UK


The briefing highlights that a significant expansion of GM mosquito releases would raise important questions about cost and effectiveness, scientific protocols, risk assessment and public consent. The not-for-profit organisation has sent the briefing to all the members of the Cayman Islands Government to highlight these issues and concerns. The briefing lists sixteen specific questions about the proposed expansion of releases, and highlights the need for proper procedures to be developed to assess costs, effectiveness and risks.
“A lot more public information is required before larger trials of GM mosquitoes can even be considered”, said Dr Helen Wallace, Director of GeneWatch UK. “There are still yawning gaps in what is known about the consequences of large scale releases, and no clear procedures for how effectiveness or risks will be assessed. We still don’t know the costs of rolling out this programme or who is going to be paying for it”.
GeneWatch UK is a not-for-profit organisation which aims to ensure that genetic science and technologies are used in the public interest, and that the public have a say about their use.
Oxitec’s Genetically Modified Mosquitoes: Ready to roll out? GeneWatch March 2017
The UK company Oxitec, now owned by US company Intrexon, first conducted experimental open releases of genetically modified (GM) mosquitoes in the Cayman Islands in 2008-09. Experimental releases re-started in the Cayman Islands in 2016 and there are plans to expand these releases to a larger scale roll-out in 2017.1
A significant expansion of GM mosquito releases raises important questions about cost- effectiveness, scientific protocols, risk assessment and public consent.
This briefing summarises the questions and concerns about these plans.
Key questions are:
Is this technology effective? How much would it cost and is it cost effective?
Are the Cayman Islands’ regulations adequate?
Have alternatives been properly considered?
Background
Oxitec’s patented technique for genetically modifying insects is known as RIDL (Release of Insects carrying a Dominant Lethal genetic system). All the company’s open field experiments to date involve its OX513A strain of the Aedes aegypti mosquito, which is genetically engineered to contain a red fluorescent marker and the RIDL ‘conditional lethality’ trait. The mosquitoes are genetically engineered to die at the larval stage in the absence of the antibiotic tetracycline, which acts as a chemical switch to allow breeding in the laboratory.
Oxitec’s male OX513A GM mosquitoes are intended to mate with wild females and produce offspring which die as larvae. Repeated releases of many millions or billions of GM males, vastly outnumbering the wild male mosquito population, are intended to reduce the total adult population of mosquitoes over time, as many of the GM offspring fail to survive to adulthood.
The GM mosquitoes released in the experiments are of the Aedes aegypti species, which transmits the tropical diseases dengue fever, zika and chikungunya.
The UK company Oxitec, now owned by US company Intrexon, has conducted experimental open releases of genetically modified (GM) mosquitoes in the Cayman Islands, Malaysia, Brazil and Panama. Oxitec’s releases of GM mosquitoes in Panama and Malaysia have ceased, due to concerns about costs, effectiveness and risks. Further proposed trials in the USA (in Key Haven, Florida Keys) have been halted while a new site and new authorisation is sought, following a local vote against the trials and the threat of legal action.2 The Food and Drug Administration (FDA) authorisation for the Key Haven trials has therefore been withdrawn.3,4 In Brazil, trials continue in the city of Piracicaba, but commercial releases have yet to be approved by the Brazilian health authority, ANVISA, which wants to see evidence of benefits to health before giving its approval, in line with recommendations from the World Health Organisation (WHO).5,6,7
Questions and concerns about open releases of Oxitec’s GM mosquitoes are outlined below.
1.Is Oxitec’s technology effective?
2.
Oxitec has made repeated claims in public materials and press releases that its releases of GM mosquitoes can suppress wild Aedes aegypti mosquito populations by 90% or more. For example, the Oxitec and MRCU public leaflet used in the Cayman Islands states “Each area [where GM mosquitoes have been released] achieved a reduction in mosquitoes of more than 90%” and shows a map with the Cayman Islands (96%), Panama (93%) and three trials in Brazil (Itaberaba, 93%, Mandacaru 99%, Pedra Branca 92%). However, there are a number of serious problems with these claims, which are discussed further below.
Further, there is as yet no evidence from any country that releases of GM mosquitoes can reduce the incidence or harm to health caused by zika, dengue or chikungunya.
No results have yet been published from Oxitec’s 2016 trials in Grand Cayman, which reportedly involved the release of more than 6 million male GM mosquitoes.8
The results of these trials and the company’s previous experiments need to be examined critically, before any decision is made to scale-up and roll out the releases across Grand Cayman. In particular, the following issues need to be considered.
1.1.1 Oxitec’s claims of reduced mosquito populations are not based on good science
During all its experiments, Oxitec has used traps which catch adult males (called BG traps) and egg traps (ovitraps) to try to measure the effects of its releases on the wild population of mosquitoes. However, there are problems with how Oxitec interprets its data and there is no direct evidence of a fall in the population of biting female mosquitos, which transmit disease.
The lead author of Oxitec’s paper reporting Oxitec’s results from Itaberaba in Brazil is Danilo Carvalho, who was reported by Al Jazeera in November 2016 as questioning Oxitec’s claims and methods (cited above).
Only one of Oxitec’s published papers include the raw data for their calculations. This is the paper which reports the results from Itaberaba.10 These results have been the subject of a critique published in the Lancet Global Health.11 As shown in the Appendix to this paper, Oxitec’s claim of 95% adult suppression in this trial is based only on captured adult male, not adult female mosquitoes. This means no conclusions can be drawn about the effect of the releases on the number of adult biting female mosquitoes which transmit disease. The start and end time for the claimed reductions are chosen by Oxitec to give the most favourable results. Further, there is no control area with which to compare the numbers of adult males, although controls are provided for the egg traps.
Insufficient data is provided in any of the papers to draw definitive conclusions about the impacts of the releases on wild mosquito populations. One issue is that wild mosquitoes may simply move to neighbouring areas to mate, once the GM mosquito releases become high enough. In the 2009 Cayman Islands experiments, the number of wild Aedes aegypti mosquito eggs, measured using egg traps (ovitraps), was observed to increase in the neighbouring control area as the population in the release area decreased (Figure 2c).12 The same effect can be seen in Oxitec’s experiments in Itaberaba, which compare ovitrap data from the control area with data from adult male traps in the release area (Figure 2D).13 Oxitec has assumed that the difference between the number of wild eggs counted in control area and the number of eggs or adult males in the release area was caused by the desired population suppression effect where the GM mosquitoes were released. However, it could alternatively mean that more Aedes aegypti eggs were laid in the control area bordering the releases because wild males and/or females moved into the control area from the target area to move away from the releases. This would mean the difference between the ovitraps in the control areas and the data from the release areas was caused (wholly or in part) by more eggs being laid in the control area, rather than by mosquitoes in the release area dying off. If so, Oxitec’s calculations of a reduction in the wild mosquito population would be incorrect.
In Panama, the experiments were different and compared ovitrap data in three different villages: therefore there are no data from Panama about whether more eggs were laid neighbouring the release site.14 Although Oxitec has done some further experiments elsewhere in Brazil, the results of these have not been published. Thus it is impossible to draw definitive conclusions about suppression of the wild population of Aedes aegypti mosquitoes from any of Oxitec’s published papers. In particular, there is no direct evidence of a fall in the number of biting females.
1.1.2 Release ratios of GM to wild mosquitoes are extremely high
One measure of the success of population suppression is the “release ratio” needed i.e. the ratio of released GM male mosquitoes to wild male mosquitoes. However, Oxitec’s published papers do not allow the release ratios to be easily determined. An unpublished report from Oxitec’s experiments in Itaberaba, Brazil suggests that release ratios there reached up to 54 to1.15 More than half a million mosquitoes a week were produced during the late phase of these experiments and the releases were concentrated in a small area of houses less than 500m by 200m.
In its first experiments in the Cayman Islands, Oxitec had to significantly increase its releases of GM mosquitoes, from the expected 3,150 males per hectare per week to about 14,000 per hectare per week, targeted on a small 16 hectare area, in order to achieve the claimed population suppression effect. When local residents complained about the nuisance caused by the very large number of mosquitoes, Oxitec halved the number of adults released and deployed about 5,600 GM pupae in cages spaced 70-90m apart across the site three times a week (see supplementary material to Harris et al., 201216). Oxitec’s computer modelling of the results from the 2009 Cayman Islands predicts that that releases of 7 million GM mosquitoes a week, in an initial phase, would be needed to suppress a population of only 20,000 wild mosquitoes (10,000 males), followed by releases of 1.9 million GM mosquitoes a week for long-term suppression, if a mixture of pupal and adult releases are used, or 2.8 million a week if only adults are released.17 The authors of the paper admit that in the real world, where mosquito populations are more complicated, higher numbers might be needed.
Oxitec has chosen to release its GM mosquitoes under favourable conditions in small areas, which may not apply when the trials are scaled up.
1.1.3 It is not clear if a reduction in Aedes aegypti numbers will lead to any benefit to health
Even if suppression of the wild population of Aedes aegypti mosquitoes is successful, this might not lead to a reduction in the harm caused by tropical diseases. There are several important issues:
(i) Disease transmission thresholds are not well known and even a small number of mosquitoes may still lead to transmission of disease;
(ii) More than one species can transmit zika, dengue and chikungunya. Non-target species, such as Aedes albopictus, will not be reduced by the releases and might possibly increase due to reduced competition, particularly in the longer term;
(iii) There is a complex relationship between human immunity and infection, so in some circumstances reducing infection can lead to reduced immunity and hence a rebound in cases of disease;
(iv) There are several mechanisms through which the technology could become less effective over time (for example, if the GM mosquitoes evolve resistance to the killing mechanism), making a rebound in disease more likely.
There has been no monitoring of the impacts on dengue fever, zika or chikungunya of Oxitec’s GM mosquito releases in any country, despite a scientific consensus that assessing impacts on disease is essential to assess the efficacy of new technologies.22,23 Oxitec and its research partners in Brazil have both admitted that the experiments there (the largest ones conducted) are inadequate to assess the impacts on disease.24,25
Oxitec has claimed that the World Health Organisation (WHO)’s Vector Control Advisory Group (VCAG) has “issued a positive recommendation in support of Oxitec’s self-limiting mosquito (OX513A)”.26 However, the relevant VGAC statement in fact says: “Full-scale programmatic deployment is not currently recommended for any of the five new potential tools reviewed by VCAG. However, the VCAG recommended the carefully planned pilot deployment under operational conditions of two tools (Wolbachia-based biocontrol and OX513A transgenic mosquitoes) accompanied by rigorous independent monitoring and evaluation”.27 More specifically the VCAG states that Randomised Controlled Trials (RCTs) “with epidemiological outcomes should be carried out to build evidence for routine programmatic use of OX513A Aedes against Aedes-borne diseases”. This advice is at odds with Oxitec’s claims that its technology is already ready for deployment.
Aedes albopictus is another species of mosquito which is found in the Cayman Islands and which can also transmit dengue, chikungunya and zika.28,29,30,31 Although this species is currently found in relatively small numbers in the Cayman Islands, it is an invasive species which has spread worldwide and has for example competitively displaced Aedes aegypti in most of Florida and in Bermuda.32,33 Aedes albopictus has been responsible for concurrent epidemics of dengue and chikungunya in some countries and its presence can also extend the dengue season and perhaps introduce new viruses.34,35,36,37,38,39 Non-target species, such as Aedes albopictus, will not be reduced by releases of GM Aedes aegypti and might possibly increase if population suppression of Aedes aegypti is successful, due to reduced competition for resources, such as breeding sites. This risk increases with larger scale, longer-term experiments. Brazilian experts have warned that dengue may mutate so that
Aedes albopictus becomes a more important dengue vector in such circumstances.40 The potentially devastating effect of a single mutation in the virus has already been observed with chikungunya.41
In the case of zika, some scientists have argued that Culex species of mosquitoes, which are common in the Cayman Islands and elsewhere, may also play an important role in transmission of disease.4243,44 Although the evidence is not definitive (and some scientists have found that Culex species do not appear to transmit zika in some regions45,46,47) at a meeting in Florida in October 2016, three independent groups from Canada, China, and Brazil presented and discussed laboratory and field data strongly suggesting that the southern house mosquito, Culex quinquefasciatus, also known as the common mosquito, is highly likely to be a vector for zika in certain environments.48 If this is the case, attempting to reduce zika transmission by targeting Aedes aegypti may be the wrong approach.
2. Costs and cost-effectiveness
The Cayman Islands’ 2016/17 budget does not make clear how the proposed releases will be funded. The total budget for mosquito control is projected to increase from $5,797,106 in 2015/16 to $9,609,900 for the 18 month period from 1 July 2016 to the end of 2017 (of which $6,406,600 is to be spent in 2017)49. There is a breakdown of this figure in the Annual budget statements, covering seven areas (larviciding; adulticiding; the MRCU’s call-out service; scientific advice; the education programme; non-chemical control, such as draining swamps; and disease prevention and control, including surveys).50 Although this document includes a commitment to “Reduce the abundance of Aedes aegypti mosquitoes by implementing a sterile male release program based on the Oxitec RIDL genetically modified mosquito” (page 165), the budget breakdown does not appear to include any costs for the production or release of Oxitec’s GM mosquitoes.
In 2011, Oxitec published a paper claiming that its technology is cost effective at preventing dengue.51 The computer modelling in this paper was conducted before any experimental results were published and is therefore out of date. The numbers of GM mosquitoes that would be needed to prevent a single case of disease remains speculative due to the problems discussed above, and (at best) appears to be much higher than Oxitec originally supposed, even if disease prevention is achievable (which is still unknown). This paper also contains some estimates of costs, based on the costs of constructing and operating facilities to produce sterile insects using irradiation (the sterile insect technique, SIT). Costs are given in US dollars at 2008 prices. The cost of construction of a SIT facility varies considerably from $50,000 to $26 million. The cost of production of sterile insects is given as a mean of $813 per million insects (range $172 to $1639 per million insects). In Brazil, cost of production of irradiated SIT Medflies ranges from $400 to $700 for every million flies released and experts question whether Oxitec’s GM flies could be produced any cheaper.52 Comparable costs are not available for mosquitoes. However, Oxitec’s new GM mosquito factory in Piracicaba, which commenced construction in June 2016, is expected to cost £2.5m to £3m according to the company’s accounts.53 The factory aims to produce 60 million GM mosquitoes per week.54 However, prices are not given and Oxitec’s 2015 accounts state: “It may be some time before the Company’s investments in Brazil lead to a self- sustaining cash flow”.
The cost of production of Oxitec’s GM mosquitoes may not be directly comparable to the production of irradiated insects for use in SIT programmes. A few press reports have provided financial figures relating to GM mosquito production. In October 2016, Science reported: “Oxitec’s mosquitoes are currently more expensive. The Piracicaba expansion will cost the city roughly $1.1 million over 2 years—some $10 per person in the treated area— about half of which will come out of the existing mosquito control budget. Oxitec itself is paying even more than that, says Slade, but it’s too early to tell how much the mosquitoes will cost if they are reared on a much larger scale. “It’s only when you roll up your sleeves and build a factory that you know what your costs are.””. If the cost is the same per person for Grand Cayman (population of 52,601) as the (subsidised) cost is in Piracicaba, this would amount to $526,010 for experimental control of Aedes aegypti over a two year period: however, it is important to note this is not the full cost of production (which has not been reported), nor is it known whether the expansion is sufficient to prevent disease. In 2014, the release of 300,000 GM mosquitoes in Panama was reported to have cost $620,000 (more than $2 per mosquito).55 At this price, releasing 7 million GM mosquitoes a week (if, as predicted, this is necessary to suppress a wild population of 20,000 mosquitos) would cost considerably more: $14.5 million a week. Alternatively, if we use the fact that Oxitec released 14,000 GM mosquitoes per hectare per week towards the end of its first Grand Cayman experiments, this would suggest that 273 million GM mosquitoes per week might be needed to cover the whole island (19,500 ha). At the reported Panama prices, this would cost more than $564 million a week; at a cost of $500 per million mosquitoes (comparable to SIT), it would cost $136,500 a week (more than $7 million a year if the releases were ongoing and could not be reduced). Any of these options would be likely to be prohibitively expensive, but much more clarity on costs is needed, due to the large variation in the estimates of both numbers needed to be released and production costs, and the absence of any confirmed published figures.
There is an important distinction to be made between Oxitec’s proposed commercial service and alternative approaches to vector control, which are normally developed and conducted on a non-commercial basis.
Any costs would be an addition to the mosquito control budget, as existing control measures, many of which focus on controlling other species, would also need to be maintained. Further, it is currently unknown whether releasing even very large numbers of GM mosquitoes would be sufficient to have an impact on the relevant diseases. Even if GM mosquitoes can reduce disease, it is hard to estimate the numbers of GM mosquitoes that would be needed, based on the results of Oxitec’s experiments discussed above. If population suppression did occur and had a positive effect, releases would need to continue indefinitely as cessation might lead to a rebound in the cases of disease (assuming the releases remained effective over time). This is the basis of Oxitec’s business model, which requires a subscription to an ongoing service. It is also unknown whether the numbers released could be reduced over time, or whether they might need to be increased (e.g. due to resistance developing), or supplemented with other measures (due to other species such as Aedes albopictus becoming more of a problem). In fact, in its application to release GM Aedes aegypti mosquitoes in the Cayman Islands, Oxitec assumes that future releases of GM Aedes albopictus will also be needed.56 Oxitec’s GM Aedes albopictus have never been tested in open air releases, so it is unclear whether this approach would work, or how much it would cost.
Since the health of members of the public should not be put at risk, clear protocols are needed to show how GM mosquito releases can be combined with conventional vector control measures which tackle all relevant species. This will add to costs, as measures such as the use of larvicides, adulticides and removal of breeding sites will harm or destroy released GM mosquitoes as well as the wild population.
3. Are the Cayman Islands’ regulations adequate?
At the time of the first experimental releases of Oxitec’s GM mosquitoes in the Cayman Islands in 2009-2010, the company was criticised because no biosafety law had been implemented and no risk assessment had been published or been subject to a public consultation.58,59 People were not properly informed that the mosquitoes were genetically modified and were wrongly told that they were sterile.
Subsequently, the National Conservation Council (NCC) was established under the National Conservation Law 201360, which states that its Director may “develop criteria for determining whether wild populations or proposed introductions of alien or genetically altered species might cause harm to any of the natural resources of the Islands and procedures for regulating and controlling such populations and introductions” (Article 6(2)(k)). Article 35 requires anyone who wishes to introduce or release in any part of the Islands a live or viable specimen of an alien or genetically altered species to apply to the Council under this Law for a permit to do so. The Chief Agricultural and Veterinary Officer must also consult the NCC when considering whether to permit and import of a genetically altered species.
In 2016, new experimental releases were the subject of a legal challenge through judicial review. The judge found that the NCC and the Department of Environment considered the potential risks before granting approval, and found that there was not a failure to consult with the public before the council’s decision.61,62 However, the judge also advised that the Department of Environment and the NCC begin to develop the “criteria, procedures and subsidiary legislation” for determining whether the introduction of alien or genetically modified species might cause any harm to natural resources and for regulating and controlling such populations and introductions.
In October 2016, the Cayman Compass reported that the NCC had prepared a draft policy covering requirements for environmental impact assessments for genetically modified organisms.63 However, the draft policy has not been published or put out for public consultation. This report also stated that the Mosquito Research and Control Unit (MRCU) had been granted permission to vary the terms of its permit, to allow it to transfer Oxitec’s GM mosquito pupae within sealed devices to a new insectary within the grounds of the MRCU prior to release, in order to increase the production and release rate during wet season. The permit was also altered to allow for an additional kilogram of eggs to be imported, increasing the total from 1.65 to 2.65kg, although the restriction to release 22 million mosquitoes remains part of the permit conditions.
In evidence to the court, the relevant authorities relied heavily on the existence of an environmental risk assessment issued by the US Food and Drug Administration (FDA) for proposed releases of Oxitec’s GM mosquitoes in the Florida Keys. However, this FDA authorisation has since been withdrawn.64,65 Further, the risk assessment applied to a small initial trial in different habitat, and the USA has no specific guidance on risk assessments for GM mosquitoes and is not a member of the Convention on Biological Diversity or the Cartagena Protocol on Biosafety (CPB).
The National Conservation Law 2013 gives effect to a number of conventions, including the Global Convention on Biological Diversity (CBD). The Cartagena Protocol on Biosafety (CPB) to the Convention on Biological Diversity is an international agreement which aims to ensure the safe handling, transport and use of genetically modified organisms (GMOs), known as living modified organisms (LMOs) under the Protocol.
Developing the necessary criteria, procedures and subsidiary legislation to properly assess the risks of scaled up releases, and regulate the use of this technology, is not a trivial task. Guidance published by the European Food Safety Authority (EFSA) outlines the evidence that Oxitec would need to provide for its GM mosquitoes to be placed on the EU market (placing on the market means making available to third parties, whether in return for payment or free of charge).66 Pages 73 to 107 of the EFSA Guidance provide details on specific areas of risk for GM insects. Under the CPB, the Ad Hoc Technical Expert Group (AHTEG) on Risk Assessment and Risk Management has also produced Guidance on the Risk Assessment of Genetically Modified Mosquitoes.67 In addition, the recommendations of the WHO’s Vector Control Advisory Group (VCAG) will need to be implemented, including the need for proper scientific protocols to assess the impacts of the proposed releases on all relevant diseases and the need for rigorous independent monitoring and evaluation.
The risks of any trial increase as the numbers are scaled up. Issues include:68,69
The survival and spread of GM mosquitoes, including biting females, and their
impacts on the environment and human health. Scaling up releases means that greater numbers will survive to adulthood, and these numbers may increase if the GM mosquitoes encounter sufficiently high levels of tetracycline in the environment to deactivate the genetic killing mechanism, or if they evolve resistance to it.
Impacts of the use of a non-native strain of mosquito, which may introduce new traits into the wild mosquito population, including different disease-transmission properties.
Disposal of the antibiotic tetracycline used to breed the GM mosquitoes in the lab, and the issue of whether released GM mosquitoes will spread antibiotic resistant bacteria into the environment.
Impacts on other species, including the question of whether population suppression of Aedes aegypti mosquitoes could lead to an increase in numbers of Aedes albopictus mosquitoes, or other disease-transmitting species.
Questions regarding the impact of the releases on the spread of tropical diseases, including efficacy and risks (including impacts on immunity).
Larger scale releases also increase the risk of problems such as the release of biting females or GM males which have evolved resistance to the killing mechanism and can breed successfully; or the release of mosquitoes which have been contaminated with infectious disease e.g. if they have been accidentally fed with infected animal blood, or if wild infected mosquitoes have somehow entered the insectary. Oxitec currently feeds its GM insects on horse blood from the UK, which will not be infected with tropical diseases which are not present in that country. However, scaling up production of GM mosquitoes in the Cayman Islands may mean a new supply of blood for feeding will be needed, and testing for infection will become essential.
It is widely recognised that fully informed consent from the public is needed for releases of genetically modified mosquitoes.70,71 Fully informed consent requires an opportunity for prior consultation on the risk assessment, as the public must be properly informed about the risks.
Fully informed consent to medical research is a requirement of the World Medical Association’s Helsinki Declaration (which covers the ethical responsibilities of medical professionals).72 For example, all medical research involving human subjects must be preceded by careful assessment of predictable risks and burdens to the individuals and groups involved in the research in comparison with foreseeable benefits to them and to other individuals or groups affected by the condition under investigation (Article 17); the design and performance of each research study involving human subjects must be clearly described and justified in a research protocol (Article 22); the study must be approved by an ethics committee (Article 23) and participants must be fully informed about the study, including potential risks (Article 26). There must therefore be public recognition that the releases are still experimental, and are not a proven approach to tackling the risk of zika, dengue or chikungunya.
4. Have alternatives been properly considered?
The World Health Organisation’s Strategic Response Plan for Zika includes an objective to prevent adverse health outcomes associated with Zika virus infection through integrated vector management, risk communication and community engagement.74 The approach includes:
● implementing integrated vector management (IVM) to efficiently and judiciously use resources, defined as “a rational decision-making process for the optimal use of resources for vector control”; ● targeting all life stages of the Aedes mosquito: egg, larva/pupa and the adult; ● reducing the risk of sexual transmission and other possible routes of transmission; ● coordinating, collaborating and partnering with stakeholders from government (municipalities, ministries of education, health, social services, water and sanitation, etc.) and civil society (NGOs, private sector, faith-based associations, churches, etc.); ● engaging and empowering communities, private sectors, etc. in mosquito control and prevention behaviours at the environmental, household, schools, businesses, personal levels, etc.; and ● developing relevant risk communication and behaviour change strategies and materials.
The WHO zika strategy also aims to fast track and scale up the research, development and availability of Aedes mosquito control tools, diagnostic tests and vaccines.
For dengue, the WHO notes that one vaccine has already been licensed, and five more are under development.75 Vector control measures include:
preventing mosquitoes from accessing egg-laying habitats by environmental management and modification;
disposing of solid waste properly and removing artificial man-made habitats;
covering, emptying and cleaning of domestic water storage containers on a weekly
basis;
applying appropriate insecticides to water storage outdoor containers;
using of personal household protection such as window screens, long-sleeved
clothes, insecticide treated materials, coils and vaporizers;
improving community participation and mobilization for sustained vector control;
applying insecticides as space spraying during outbreaks as one of the emergency
vector-control measures.
The WHO states that active monitoring and surveillance of vectors should be carried out to determine effectiveness of control interventions.
Any decision about future use of Oxitec’s GM mosquitoes must consider all the alternatives and whether the money could be better spent.
Conclusions and recommendations
Before any further open releases of GM mosquitoes are considered:
Decision-makers in the Cayman Islands must thoroughly consider whether releases
of Oxitec’s GM mosquitoes are effective; how much they cost and whether they are cost-effective; and the existence of alternatives. The relevant information, including the results of Oxitec’s 2016 trials and estimates of future pricing, should be made publicly available.
The National Conservation Council (NCC) and Department of Environment must develop the necessary criteria, procedures and subsidiary legislation to properly assess the risks of scaled up releases, and regulate the use of this technology. Policies should be subject to public consultation;
The recommendations of the WHO’s Vector Control Advisory Group (VCAG) should be implemented, including: the need for proper scientific protocols to assess the impacts of the proposed releases on all relevant diseases; and the need for rigorous independent monitoring and evaluation;
Much more openness is needed about the proposed trials, including detailed answers to specific questions.
In particular, a clear process needs to be set out for decision-making on proposed future trials. Some specific questions that need to be answered are:
What are the scientific protocols for the proposed future trials, including: epidemiological and population endpoints to measure impact on adult female mosquito populations and risk of disease (for zika, dengue and chikungunya); and the protocols for combining GM mosquito releases with other vector control measures, including spraying?
How will independent evaluation and monitoring of the proposed trials be implemented? How will monitoring be funded?
What is the proposed procedure for environmental risk assessment and will the draft policy and regulations be published and be subject to public consultation?
Once the procedure is established, will future environmental risk assessments be subject to public consultation?
What will the procedure be for amending or expanding licences?
What is the purpose of the amendment to the existing permit reported in October
2016, which allows GM pupae to be transferred to a new insectary? Does this mean there have been breeding problems, and what are the implications for the proposed experiments?
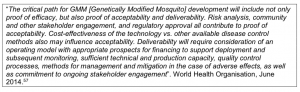
What are the expected future costs of the trials and future annual costs, and how is this intended to be funded? As well as the production costs of the GM mosquitoes, what are the costs of sufficient technical and production capacity, quality control processes, methods for management and mitigation in the case of adverse effects, and ongoing stakeholder engagement?
Will the results of the current trials be published in a peer reviewed journal before a decision to expand the trials is taken?
Will there be any independent scrutiny of Oxitec’s trial results to date?
What evidence currently exists regarding the presence and role of Aedes aegypti and other mosquito species in transmitting zika, dengue and chikungunya on the Cayman Islands? For example, will monitoring trap data and mosquito population estimates be published? Will locations of cases of these diseases be published, including the locations of the non-published zika cases of 2016, as well as evidence regarding disease transmission for all species (including Aedes albopictus and Culex species)?
What measures will be taken to assess and address potential impacts of GM mosquito trials on other mosquito species? Will a proper study be undertaken of the potential impacts of other species on disease, prior to any decisions on undertaking further trials?
How will other potential risks be more thoroughly assessed? For example: will feeding trials be used to test the risk of consumption of GM mosquitoes by other species; will the presence of antibiotic resistant bacteria be investigated before GM mosquitoes are released; will potential breeding sites, including septic tanks, be surveyed for tetracycline contamination; will the GM mosquitoes be tested for disease transmission properties before they are released?
What quantities of the antibiotic tetracycline will be used during the GM mosquito breeding process, how will this be regulated, and how will disposal be controlled and monitored?
If new releases do take place, how will impacts on other mosquito species be monitored, in the short- and long-term?
Will protocols be developed and published to minimise and detect the risk of the accidental release of biting female mosquitoes; the use of contaminated feed; and the evolution or accidental introduction of GM mosquitoes which are resistant to the killing mechanism?
Will an economic report be published prior to any decision, assessing the potential effects of long-term wide-scale releases of GM mosquitoes on the tourism industry?
References
GeneWatch UK
86 Dedworth Road, Windsor, Berkshire, SL4 5AY, UK Phone: 0330 0010507 Email: [email protected] Website: www.genewatch.org Registered in England and Wales Company Number 3556885
References
1 Zika GM project likely to expand. The Cayman Reporter. 15th January 2017. http://www.caymanreporter.com/2017/01/13/zika-gm-project-likely-expand/ 2 Florida Needs a New Place to Release Genetically-Modified Anti-Zika Mosquitoes. MotherBoard. 21st November 2016. http://motherboard.vice.com/read/florida-needs-a-new-place-to-release- genetically-modified-anti-zika-mosquitoes 3 Victory! GE mosquitoes will not be let loose on Florida community. Friends of the Earth, Center for Food Safety Press Release. 7th December 2016. http://www.foe.org/news/archives/2016-12-victory- ge-mosquitoes-will-not-be-let-loose-on-florida-community 4 FDA backs down from releasing genetically modified mosquitoes after lawsuit threatened. Florida Record. 8th January 2017. http://flarecord.com/stories/511066542-fda-backs-down-from-releasing- genetically-modified-mosquitoes-after-lawsuit-threatened 5 Anvisa decide que mosquito transgênico é objeto de regulação sanitaria [Anvisa decides that transgenic mosquitoes are subject to sanitary regulation]. ANVISA. 12th April 2016. http://portal.anvisa.gov.br/noticias/-/asset_publisher/FXrpx9qY7FbU/content/anvisa-decide-que- mosquito-transgenico-e-objeto-de-regulacao- sanitaria/219201/pop_up?_101_INSTANCE_FXrpx9qY7FbU_viewMode=print&_101_INSTANCE_FXr px9qY7FbU_languageId=en_US 6 Anvisa decide que mosquito transgênico é objeto de regulação sanitaria. REDE Brasil Actual. 12th April 2016. http://www.redebrasilatual.com.br/saude/2016/04/anvisa-decide-que-mosquito- transgenico-e-objeto-de-regulacao-sanitaria-7405.html 7 WHO (2016) Mosquito (vector) control emergency response and preparedness for Zika virus. 18th March 2016. http://www.who.int/neglected_diseases/news/mosquito_vector_control_response/en/ 8 Zika GM project likely to expand. The Cayman Reporter. 15th January 2017. http://www.caymanreporter.com/2017/01/13/zika-gm-project-likely-expand/ 9 Can Genetically Modified Mosquitoes Eradicate Malaria? TechKnow, Al Jazeera, 29th November 2016. http://www.aljazeera.com/programmes/techknow/2016/11/genetically-modified-mosquitoes- eradicate-malaria-161129075454200.html 10 Carvalho DO, McKemey AR, Garziera L, Lacroix R, Donnelly CA, Alphey L, … Capurro ML (2015) Suppression of a Field Population of Aedes aegypti in Brazil by Sustained Release of Transgenic Male Mosquitoes. PLOS Neglected Tropical Di. 9(7). http://dx.doi.org/10.1371/journal.pntd.0003864 11 Boëte C., Reeves RG (2016). Alternative vector control methods to manage the Zika virus outbreak: more haste, less speed. The Lancet Global Health, 4(6), e363. https://doi.org/10.1016/S2214-109X(16)00084-X 12 Harris AF, McKemey AR, Nimmo D, Curtis Z, Black I, Morgan SA, Oviedo MN, Lacroix R, Naish N, Morrison NI, Collado A, Stevenson J, Scaife S, Dafa’alla T, Fu G, Phillips C, Miles A, Raduan N, Kelly N, Beech C, Donnelly CA, Petrie WD, Alphey L (2012) Successful suppression of a field mosquito population by sustained release of engineered male mosquitoes. Nat. Biotech., 30(9), 828–830. 13 Carvalho DO, McKemey AR, Garziera L, Lacroix R, Donnelly CA, Alphey L, … Capurro ML (2015) Suppression of a Field Population of Aedes aegypti in Brazil by Sustained Release of Transgenic Male Mosquitoes. PLOS Neglected Tropical Di. 9(7). http://dx.doi.org/10.1371/journal.pntd.0003864 14 Gorman K, Young J, Pineda L, Márquez R, Sosa N, Bernal, D., … Cáceres, L. (2016). Short-term suppression of Aedes aegypti using genetic control does not facilitate Aedes albopictus. Pest Management Science, 72(3), 618–628. https://doi.org/10.1002/ps.4151 15 PAT (2012) Transgenic Aedes Project Progress Report, Feb 2011-Mar 2012. 16 Harris AF, McKemey AR, Nimmo D, Curtis Z, Black I, Morgan SA, Oviedo MN, Lacroix R, Naish N, Morrison NI, Collado A, Stevenson J, Scaife S, Dafa’alla T, Fu G, Phillips C, Miles A, Raduan N, Kelly N, Beech C, Donnelly CA, Petrie WD, Alphey L (2012) Successful suppression of a field mosquito population by sustained release of engineered male mosquitoes. Nat. Biotech., 30(9), 828–830. 17 Winskill P, Harris AF, Morgan SA, et al. (2014) Genetic control of Aedes aegypti: data-driven modelling to assess the effect of releasing different life stages and the potential for long-term suppression. Parasites & Vectors 7(1):68. 18 The Guidance Framework for testing genetically modified mosquitoes. WHO/TDR and FNIH. June 2014. http://www.who.int/tdr/publications/year/2014/guide-fmrk-gm-mosquit/en/ 19 Can Genetically Modified Mosquitoes Eradicate Malaria? TechKnow, Al Jazeera, 29th November 2016. [At 16:20]. http://www.aljazeera.com/programmes/techknow/2016/11/genetically-modified- mosquitoes-eradicate-malaria-161129075454200.html
20 When science bites back.Science Line. 28th March 2014. http://scienceline.org/2014/03/when- science-bites-back/ 21 Leal W S (2016). Zika mosquito vectors: the jury is still out. F1000Research,5. https://doi.org/10.12688/f1000research.9839.1
22 James S, Simmons CP, James AA (2011) Mosquito Trials. Science, 334(6057), 771-772. 23 Wolbers, M., Kleinschmidt, I., Simmons, C. P., & Donnelly, C. A. (2012). Considerations in the Design of Clinical Trials to Test Novel Entomological Approaches to Dengue Control. PLoS Negl Trop Dis, 6(11), e1937. 24 Brazil to unleash GM-mosquito swarms to fight dengue. New Scientist. 23rd July 2014. http://www.newscientist.com/article/dn25936-brazil-to-unleash-gmmosquito-swarms-to-fight- dengue.html#.U-s4o2NeKSr 25 Engineering Mosquitoes to Spread Health. The Atlantic. 13th September 2014. http://www.theatlantic.com/health/archive/2014/09/engineering-mosquitoes-to-stop-disease/379247/ 26 World Health Organization (WHO) Issues Positive Recommendation for Oxitec’s Self-limiting Mosquito. Press Release. Oxitec. 21st March 2016. http://www.oxitec.com/world-health-organization- issues-positive-recommendation-oxitecs-self-limiting-mosquito/ 27 Mosquito (vector) control emergency response and preparedness for Zika virus. World Health Organisation. 18 March 2016. http://www.who.int/neglected_diseases/news/mosquito_vector_control_response/en/ 28 http://mrcu.ky/brief-guide-to-common-mosquitoes/ 29 Benedict MQ, Levine RS, Hawley WA, Lounibos LP (2007) Spread of the Tiger: Global Risk of Invasion by the Mosquito Aedes albopictus. Vector Borne Zoonotic Dis. 7(1):76–85. 30 Vega-Rúa, A., Zouache, K., Girod, R., Failloux, A.-B., & Lourenço-de-Oliveira, R. (2014). High Level of Vector Competence of Aedes aegypti and Aedes albopictus from Ten American Countries as a Crucial Factor in the Spread of Chikungunya Virus. Journal of Virology, 88(11), 6294–6306. 31 Miller MJ, Loaiza JR (2015) Geographic Expansion of the Invasive Mosquito Aedes albopictus across Panama – Implications for Control of Dengue and Chikungunya Viruses. PLoS Negl Trop Dis, 9(1), e0003383. 32 Britch SC, Linthicum KJ, Anyamba A, Tucker CJ, Pak EW; Mosquito Surveillance Team (2008) Long-term surveillance data and patterns of invasion by Aedes albopictus in Florida. J Am Mosq Control Assoc. 24(1):115-20. 33 Kaplan L, Kendell D, Robertson D, Livdahl T, Khatchikian C (2010) Aedes aegypti and Aedes albopictus in Bermuda: extinction, invasion, invasion and extinction. Biological Invasions, 12 (9), 3277-3288. 34 Paupy C et al. (2010) Comparative role of Aedes albopictus and Aedes aegypti in the emergence of Dengue and Chikungunya in central Africa. Vector Borne and Zoonotic Diseases, 10(3), 259-266. 35 Ali M et al. (2003) Use of a Geographic Information System for Defining Spatial Risk for Dengue Transmission in Bangladesh: Role for Aedes Albopictus in an Urban Outbreak. The American Journal of Tropical Medicine and Hygiene, 69(6), 634-640. 36 Rezza G (2012) Aedes albopictus and the reemergence of Dengue. BMC Public Health, 12(1), 72. 37 Duncombe J, Espino F, Marollano K, et al. (2013) Characterising the spatial dynamics of sympatric Aedes aegypti and Aedes albopictus populations in the Philippines. Geospat Health. 8(1):255–265. 38 Sirisena PDNN, Noordeen F (2014) Evolution of dengue in Sri Lanka-changes in the virus, vector, and climate. Int J Infect Dis. 19:6–12. 39 Grard G, Caron M, Mombo IM, et al. (2014) Zika Virus in Gabon (Central Africa) – 2007: A New Threat from Aedes albopictus? PLoS Negl Trop Dis. 8(2):e2681. 40 Technical Opinion on Examination Request presented at the 171st Plenary Meeting of the National Technical Commission on Biosafety (CTNBio), held on April 10th, 2014 Procedure: 01200.002919/2013-77 Applicant: Oxitec do Brasil Participações Ltd. http://aspta.org.br/wp- content/uploads/2014/08/Critical-vote-GM-Mosquito-jul2014.pdf 41 Tsetsarkin KA, Vanlandingham DL, McGee CE, Higgs S (2007) A Single Mutation in Chikungunya Virus Affects Vector Specificity and Epidemic Potential. PLoS Pathog, 3(12), e201. 42 Ayres CFJ (2016) Identification of Zika virus vectors and implications for control. The Lancet Infectious Diseases, 16(3), 278–279. https://doi.org/10.1016/S1473-3099(16)00073-6 43 Guo X, Li C, Deng Y, Xing D, Liu Q, Wu Q, … Zhao T (2016). Culex pipiens quinquefasciatus: a potential vector to transmit Zika virus. Emerging Microbes & Infections, 5(9), e102. https://doi.org/10.1038/emi.2016.102
44 Guedes DRD, Paiva MHS, Donato MMA, Barbosa PP, Krokovsky L, Rocha SW dos S, … Ayres CFJ (2016). Zika virus replication in the mosquito Culex quinquefasciatus in Brazil. bioRxiv, 073197. https://doi.org/10.1101/073197 45 Hart CE, Roundy CM, Azar SR, Huang JH, Yun R, Reynolds E., … Weaver SC (2017). Zika Virus Vector Competency of Mosquitoes, Gulf Coast, United States. Emerging Infectious Diseases, 23(3), 559–560. https://doi.org/10.3201/eid2303.161636
46 Fernandes RS, Campos SS, Ferreira-de-Brito A, Miranda RM de, Silva KAB da, Castro MG de, … Lourenço-de-Oliveira, R. (2016). Culex quinquefasciatus from Rio de Janeiro Is Not Competent to Transmit the Local Zika Virus. PLOS Negl Trop Dis, 10(9), e0004993. https://doi.org/10.1371/journal.pntd.0004993
47 Huang Y-J S., Ayers VB, Lyons AC, Unlu I, Alto BW, Cohnstaedt LW, … Vanlandingham DL (2016) Culex Species Mosquitoes and Zika Virus. Vector-Borne and Zoonotic Diseases. https://doi.org/10.1089/vbz.2016.2058 48 Leal W S (2016). Zika mosquito vectors: the jury is still out. F1000Research,5. https://doi.org/10.12688/f1000research.9839.1
49 Plan and Estimates 2016/17. For the 18 month period ending 31December 2017. Cayman Islands Government. [HES 11: Mosquito Control Services,page 83]. http://www.gov.ky/portal/page/portal/pfehome/publications/2016-17-Budget-Documents/2016-17-Plan- Estimates
50 Budget Statements. 2016/17. For the 18-month period ending 31 December 2017. [Pages 201- 207] http://www.gov.ky/portal/page/portal/pfehome/publications/2016-17-Budget-Documents/2016-17- Budget-Statements 51 Alphey N, Alphey L, Bonsall MB (2011). A model framework to estimate impact and cost of genetics-based sterile insect methods for dengue vector control. PloS One, 6(10), e25384. https://doi.org/10.1371/journal.pone.0025384 52 Empresa de biotecnologia quer vender moscas transgênicas no Brasil. Folha de S. Paulo. 6th November 2016. http://www1.folha.uol.com.br/ciencia/2016/11/1829606-empresa-quer-comercializar-moscas-transgenicas- no-brasil.shtml 53 Oxitec Limited Annual Report for the Year Ended 31 December 2015. https://beta.companieshouse.gov.uk/company/04512301/filing-history 54 Oxitec Opens Large Scale Mosquito Production Facility in Brazil. October 26, 2016. http://www.oxitec.com/oxitec-opens-large-scale-mosquito-production-facility-brazil/ 55 Liberados 300 mil mosquitos transgenicos [In Spanish]. TVN-2. 9th May 2014. http://www.tvn- 2.com/nacionales/Liberados-mil-mosquitos-transgenicos_0_3931106958.html 56 Application to conduct scientific study in the Cayman Islands. July 2014 – July 2015. http://www.centerforfoodsafety.org/files/2014-application-to-doe-3_97611.pdf 57 The Guidance Framework for testing genetically modified mosquitoes. WHO/TDR and FNIH. June 2014. http://www.who.int/tdr/publications/year/2014/guide-fmrk-gm-mosquit/en/ 58 Reeves RG, Denton JA, Santucci F, Bryk J, Reed FA (2012). Scientific Standards and the Regulation of Genetically Modified Insects. PLoS Neglected Tropical Diseases, 6(1), e1502. https://doi.org/10.1371/journal.pntd.0001502 59 Enserink M (2010) GM Mosquito Trial Strains Ties in Gates-Funded Project. Science Insider. 16 November 2010. http://news.sciencemag.org/scienceinsider/2010/11/gm-mosquito-trial-strains- ties.html?ref=hp 60 The National Conservation Law, 2013 (Law 24 of 2013). Cayman Islands. http://www.doe.ky/wp- content/uploads/2010/03/NCL-Gazetted-5-Feb-2014.pdf 61 Judge explains mosquito project decision. The Cayman Compass. 27th July 2016. https://www.caymancompass.com/2016/07/27/judge-explains-mosquito-project-decision/ 62 https://caymannewsservice.com/wp-content/uploads/2016/07/Draft-Judgment-Judicial-Review-26- July16-GM-mosquito-release.pdf 63 New policy for vetting genetically modified imports. Cayman Compass. 27th October 2016. https://www.caymancompass.com/2016/10/27/new-policy-for-vetting-genetically-modified-imports/ 64 Victory! GE mosquitoes will not be let loose on Florida community. Friends of the Earth, Center for Food Safety Press Release. 7th December 2016. http://www.foe.org/news/archives/2016-12-victory- ge-mosquitoes-will-not-be-let-loose-on-florida-community
65 FDA backs down from releasing genetically modified mosquitoes after lawsuit threatened. Florida Record. 8th January 2017. http://flarecord.com/stories/511066542-fda-backs-down-from-releasing- genetically-modified-mosquitoes-after-lawsuit-threatened 66 EFSA (2013) Guidance on the environmental risk assessment of genetically modified animals. EFSA Journal 2013;11(5):3200 [190 pp.]. http://www.efsa.europa.eu/en/efsajournal/pub/3200.htm 67 Guidance on Risk Assessment of Living Modified Organisms: Risk Assessment of Living Modified Mosquitoes. Biosafety Clearing House. http://bch.cbd.int/onlineconferences/guidancedoc_ra_mosquitoes.shtml (English) or . Available as pdf in English: http://www.cbd.int/doc/meetings/bs/mop-06/official/mop-06-13-add1-en.pdf and Spanish: http://www.cbd.int/doc/meetings/bs/mop-06/official/mop-06-13-add1-es.pdf
68 Oxitec’s Genetically Modified Mosquitoes: A Credible Approach to Dengue Fever? GeneWatch UK. March 2015. http://www.genewatch.org/uploads/f03c6d66a9b354535738483c1c3d49e4/Oxitec_GWbrief_Mar15.pd f
69 GeneWatch UK comments on FDA Docket FDA-2014-N-2235: Oxitec OX513A Mosquitoes. 17th May 2016. http://www.genewatch.org/uploads/f03c6d66a9b354535738483c1c3d49e4/FDA_Oxitec_GWresponse _fin2.pdf
70 Macer, D. Ethical, legal and social issues of genetically modified disease vectors in public health. TDR (2003). http://www.who.int/tdr/publications/tdr-research-publications/seb_topic1/en/index.html 71 Macer D (2005) Ethical, legal and social issues of genetically modifying insect vectors for public health. Insect Biochemistry and Molecular Biology 35, 649-660.
72 World Medical Association. Helsinki Declaration.
http://www.wma.net/en/30publications/10policies/b3/index.html
73 Boëte C., Reeves RG (2016). Alternative vector control methods to manage the Zika virus outbreak: more haste, less speed. The Lancet Global Health, 4(6), e363. https://doi.org/10.1016/S2214-109X(16)00084-X 74 Zika Strategic Response Plan. World Health Organization. July 2016. http://www.who.int/emergencies/zika-virus/strategic-response-plan/en/
75 http://www.who.int/immunization/research/development/dengue_q_and_a/en/